Air Purifiers to limit virus spread
The effect of chronic allergies resulting in breathing difficulty led French electronics engineer Thierry Ricci to develop an air-purifying technology that has since been used in car manufacturing plants as well as by European aircraft manufacturer Airbus. Now it is being offered to hospitals, clinics, medical offices and nursing homes to reduce the airborne spread of micro-organisms, including viruses such as SARS-CoV-2, and is available in New Zealand through Opritech.
.jpg)
NatéoSanté, designer and manufacturer of the EOLIS Air Purifiers, is referenced by the French Healthcare Association in the treatment/disinfection of hospital air category. Thanks to its indoor air purification solutions, designed to meet complex and difficult sanitary requirements, NatéoSanté helps limit the spread of the virus by air.
The EOLIS Air Manager combines a unique medical grade filtration system with controllable and activatable UV-C lamps. Each unit is equipped with state-of-the-art technology that continuously measures and analyses indoor air quality, allowing you to monitor air quality and filter wear in real time.
It is the four-step filtration system that is the key to the EOLIS Air Manager. First there is a virucidal, bactericidal and acaricidal pre-filter barrier (Total Science™ TS-010 Disinfecting solution, CertifiedEN1276 and EN14675).
Then there is a very high density (VHD) carbon activated filter. After passing through this, the air is filtered by a medical grade high-efficiency particulate air (HEPA) filter which can catch particles as small as 0.1 micronmeters (Certified EN1822).
Finally, the finely filtered air passes through a photocatalysis filter + UV-C lamps. This is acombination of catalyst with UV-C germicidal lamps.
.jpg)
High intensity pulsed Ultraviolet C or UV-C light is an effective way to kill most bacteria, viruses and fungi in the air and on surfaces and requires a significantly reduced exposure time and greatly improved usable distance when compared to other methods using UVA and UVB light. UV disinfection can be applied in many situations and as part of a well-prepared plan to improve health and safety in a range of both public and private situations.
Ultraviolet disinfection technology eliminates a high percentage of pathogens both in the air and on surfaces and unlike other methods leaves no chemical residue.
The EOLIS Air Manager comes in two versions, the 600S which has a maximum airflow of 500 cubic metres per hour and the 1200S which provides 850 cubic metres of airflow an hour.
EOLIS Air Managers offer:
• an active oxygen function for deep cleaning;
• Live air quality indicator;
• Filter service indicator;
•Touchscreen control;
• Smartphone remote control (IOS and Android);
•Optional monitoring software available for the simple management of several units.
NatéoSanté EOLIS Air Managers are available in New Zealand from Opritech. For full information, call 0800 32 40 32; email: sales@opritech.co.nz — or visit www.opritech.co.nz
The full range of Opritech medical supplies may also be searched elsewhere in MEDSPEC.
Simeon launches first operating table

German operating theatre equipment specialist Simeon Medical GmbH & Co. KG has developed its first operating table — the Sim.MOVE 800 — rounding off the company’s portfolio of surgical equipment, which includes surgical lights, camera systems, a video management system, and ceiling-mounted supply units.
Simeon placed early emphasis on dialog with expert medical professionals during the development of the Sim.MOVE 800. The knowledge gained from this collaboration, combined with the company’s 20 years of experience in the surgical field, resulted in an all-new table that facilitates everyday work in the OR while increasing patient safety and comfort.
To simplify the changeover between different theatre disciplines, the Sim.MOVE 800 can be easily adapted to all conditions, making itsuitable for all common applications. It can be used in hybrid operating theatres: with its large longitudinal translation and an extended X-ray window, a change to an X-ray table is no longer necessary. Thanks to its high maximum load of 454 kg, the table can also be used for bariatric patients. The 400 mm mattress platform allows a flexible intra operative use of a C-arm without having to reposition the patient.

Thanks to an optional fifth extendable wheel in the table base, the Sim.MOVE 800 is effortlessly manoeuvrable. The optional "2-DRIVE" traction drive helps it move smoothly even under maximum load, allowing patients to be effortlessly and safely introduced to the OR through the patient airlock.
To test the real-world suitability of the Sim.MOVE 800, a comprehensive usability study was conducted at the Institute of Medical Technology at RWTH Aachen University in cooperation with the University Hospital of Aachen under the direction of Dr. Armin Janß. Particular emphasis was placed on the patient positioning display provided by "my.LIGHT" and the hand control.
"The "my.LIGHT" functions eliminate operating errors to the greatest possible extent and increase safety when using the operating table in everyday surgery. The control is quick to learn and easy to memorize. We can therefore confirm the user-friendliness of the table based on our study," says Dr. Janß.
In addition to the three most common pre-installed table positions Flex, Beach-chair and Wake-up, up to five additional positions can be stored via the memory function in the hand control. This allows users to adjust the optimal table position and orientation in just a few simple steps.
For more information on the Sim.MOVE 800, contact Simeon’s exclusive New Zealand agent, Keyport Medical Ltd., 21 The Boulevard, Te Rapa Park, Hamilton. Telephone: 07 849 3766, or 07 848 3672. After hours: 027 493 9046. Email: info@keyport.co.nz— or: sales@keyport.co.nz
Milano workstations carts & medical keyboards
_no icons.JPG)
Cubro has added customisable workstations to its comprehensive range of Milano carts and trolleys. These new lightweight workstations enable untethered productivity and the flexibility needed to support today's fast-paced medical environments.
The Milano workstations offer personalised height adjustment with intuitively placed features that support staff well-being to keep the focus on patient care. As well as the lightweight design, the Milano workstations offer a compact footprint, making them easy to use and manoeuvre. With a range of accessory combinations, Cubro can customise the Milano workstations to meet the specific needs of each unique working environment.
Key features
· Lightweight and easy to manoeuvre;
· Height adjustable with gas spring for sitting and standing positions;
· Compact design with small footprint for use in tight hospital environments;
· Smooth and easy to clean surfaces;
· Multipurpose for a variety of applications;
· Modular design allows for easy configuration and can be re-adapted as needs change;
· Laptop tray with two shelves for laptop security;
· Keyboard tray;
· Strong and durable with 5-year warranty;
· 4 x 100mm twin castors, 2 x braking.
Milano medical keyboards:
· Come in black or white;
· Adjustable backlit display (black only);
· Unique ‘clean’ button;
· USB connector cover.
Contact Cubro for more information or to arrange a trial or clinical evaluation, telephone - 0800 656 527; Email: hello@cubro.co.nz
Cane-based hollowware from Defries

In its quest to provide environmentally friendly products in its portfolio, Defries Industries is now offering the NewGen Surgical range of hollowware and needle-counters, designed to replace single-use plastic medical products with plant-based product lines, clinically developed for use in the operating room and custom procedure packs.
NewGen takes an agricultural sugar-cane by-product and upcycles it into a plastic-free healthcare product. These environmentally friendly and biodegradable products are made with 100% plant-based materials and contain no BPA, mercury, phthalates or PVC. They are coated with a biocompatible film.
“The Defries team is really excited to introduce this new plant-based product range,” says New Zealand company representative Wayne Titmus.
“We are introducing them as components in our Custom Packs and then in the coming months, will offer them as a single sterile option.”
The NewGen hollowware range comprises:
• 700ml kidney dishes
• 6L splash bowls
• 1L bowls
The needle counters from NewGen comprise 40 Count Foam Block and Double Magnet and 40-80 Count with Double Foam Block and Double Magnet. They are 95% plastic-free.
The outer box is made with bagasse, a post-agricultural by-product from sugarcane production, upcycled to create the fibre pulp. The hinges, latch, EPP foam and magnet are similar to those found in existing needle counters and made from medical grade materials.
.jpeg)
For full details on the NewGen range of biodegradable products contact Wayne Titmus, telephone: +61 448 043 275 or email him at: wayne.titmus@defries.com.au
Also check the full Defries Industries product range at www.medspec.co.nz
Surgical Clippers with twistable head
The world`s first surgical clipper with twistable shaving head is now available in New Zealand through Jackson Allison Medical & Surgical (JAMS).
The ME Medical™ Surgical Clipper is designed forsafe operation in hard-to-reach areas of the body. It offers the right blade for the right type of clipping: a Universal blade and a Neuro blade. It also minimises risk of injury with a non-slip grip and the single blade can be easily inserted with two fingers on the shaving head. The disposable blade is easily removed by pressing the eject button.
ME Medical’s Surgical Clipper offers low noise level, high efficiency, thorough shaving and are easy to clean and disinfect. They also offer high battery performance and short charging time. They are also safe to use: the guide strip to the skin-side of the single blade ensures a smooth sliding on the skin.
ME Medical™ Surgical Clippers are available from Jackson Allison, 0800 333 103, or email: enquiries@jackson-allison.co.nz
Jackson Allison offers Sharpsafe®
.jpg)
Jackson Allison Medical & Surgical has added Plascare’s range of Sharpsafe® purpose-designed disposable plastic sharps containers to its product portfolio.
Plascare is a family owned and operated business dedicated to health and environmental protection for the past 30 years. It is the licensed manufacturer and distributor of the Sharpsafe range throughout Australia. High quality and continuity of supply is what sets Sharpsafe apart from others, providing clinicians peace of mind with a dependable and safe sharps disposal solution.
In 1982 Sharpsafe was awarded the Design Council Award for excellence in design. Since then, Sharpsafe has consistently led the health industry in improving safety standards of disposable sharps containers.
Features:
• Welded construction for added strength and safety;
•Single hand operated portable sharps containers;
• Nestable containers for efficient storage and transport
• Flip top lid for temporary and final closure;
• In-mould label which cannot be removed, even by autoclaving;
• Puncture resistant base;
• Autoclavable to 135°C for 20 minutes for an empty, open container;
• Conforms to Australian, British and European standards;
• Available in a range of sizes — 200ml to 24 litre.
Plascare’s Sharpsafe range is available from Jackson Allison, 0800 333 103, or email: enquiries@jackson-allison.co.nz
Simeon adopts recyclable materials
Commitment to the environment is crucial. Simeon Medical has taken this on-board and follows strict principles for quality control
as well as environmental management in the design and manufacture of its surgical lights.

Simeon surgical lights are made from reusable materials including aluminium and glass, but do not include plastics.
These principles and actions are confirmed with certification to ISO 14001 and MDSAP.
Simeon Medical is a small manufacturing company supplying directly to the surgical market so it can react and incorporate
new developments into its products as they happen. Recent developments include the BusinessLine range and the Multicolour LED option
with a five-step selection from 3500K to 5500K all at a constant R9 across the range.
Going from strength to strength, Simeon Medical is the sole examination room, procedure room and operating theatre light supplier
for the new Intensive Care, Emergency and Surgery centre, in the Hospital of Fulda Germany. Simeon Medical has also installed
Highline OR lights, Sim.FLEX suspension arm systems and cameras in 37 operating theatres and procedure rooms
Gold Coast University Hospital, in Australia..
There is also a unique modular suspension system (Sim.FLEX) that allows changes to the configuration of the surgical lights
and accessories to be easily changed to meet future requirements.
The complete Simeon Medical range is available in New Zealand through Keyport, a Hamilton company with more than 30 years’ experience
in selling, installing and maintaining surgical lights. Keyport is confident the technical features of the Simeon Medical range are second to none.
Contact Keyport on 0800 KEYPORT (0800 539 7678) to discuss your future requirements. Email: sales@keyport.co.nz
Jackson Allison’s water-based sanitiser


Jackson Allison Medical & Surgical has a water-based skin sanitiser for the New Zealand healthcare market. It is Biospada Plus from US-based KatanTech.
KatanTech produces and markets a range of products for retail, commercial, agricultural and healthcare applications. Its products are eco-friendly and easy to incorporate in a wide variety of processes and are made to protect surfaces against almost all forms of algae, bacteria, fungi, germs, mildew, mould and viruses.
Published studies (AORN Journal 1998) report that benzalkonium chloride-based hand sanitisers demonstrate greater sustained activity than gelled alcohol gel hand sanitisers that actually became less effective with repeated use and made the skin dirtier, not cleaner due to removal of protective natural skin oils and entrapment of dead skin cells by the polymer thickeners used in the gelled alcohol products.
KatanTech’s Biospada Plus hand sanitiser incorporates Self-Aligning Polymeric Antimicrobial Durable Array (SPADA) advanced nano-scale technology that achieves durable, effective, cost-efficient and ecological anti-microbial protection.
SPADA nanotechnology works by providing a protective layer of molecules called nano-swords to the skin surface. The base layer adheres strongly to the skin molecules (or any other surface) and also attaches to other SPADA molecules in a way that keeps the ‘sword’ upright. The tip of the molecules act like the point of a sword, physically piercing the cell walls of micro-organisms, and destroying them.
Biospada Plus skin sanitiser is said to provide greater sustained anti-microbial activity than alcohol-based hand sanitisers as it isn’t removed when washing hands. Due to its unique chemistry, SPADA will not ‘leach’ off because it microscopically bonds to the surface to which it is applied.
Additionally, since SPADA molecules do not allow super bugs to be created, the antimicrobial protection this technology provides is both eco-responsible and long-lasting.
The active ingredient in SPADA nanotechnology has been approved by national and international authorities, including the New Zealand Food Safety Authority. It provides on-going protection for up to eight-hours, although it is recommended that reapplication is made every three to four hours due to the natural exfoliation of skin and hands being subject to abrasion through normal daily activity.
KatanTech hand sanitizers haveadvantages over alcohol hand sanitisers as they are non-flammable, less drying to skin, and will not stain clothing. An alcohol-free composition ensures natural oils are not stripped from the skin. It does not create micro-fissures that increase susceptibility to infection by pathogens – a common problem with alcohol-based sanitisers.
For more information on KatanTech’s Biospada Plus, contact Jo-Anne Briggs at Jackson Allison, 021 670 262 or email: enquiries@jackson-allison.co.nz
Reference
AORN Journal, 68 August 1998, pp. 239-251.
Cubro offers new patient transfer chairs
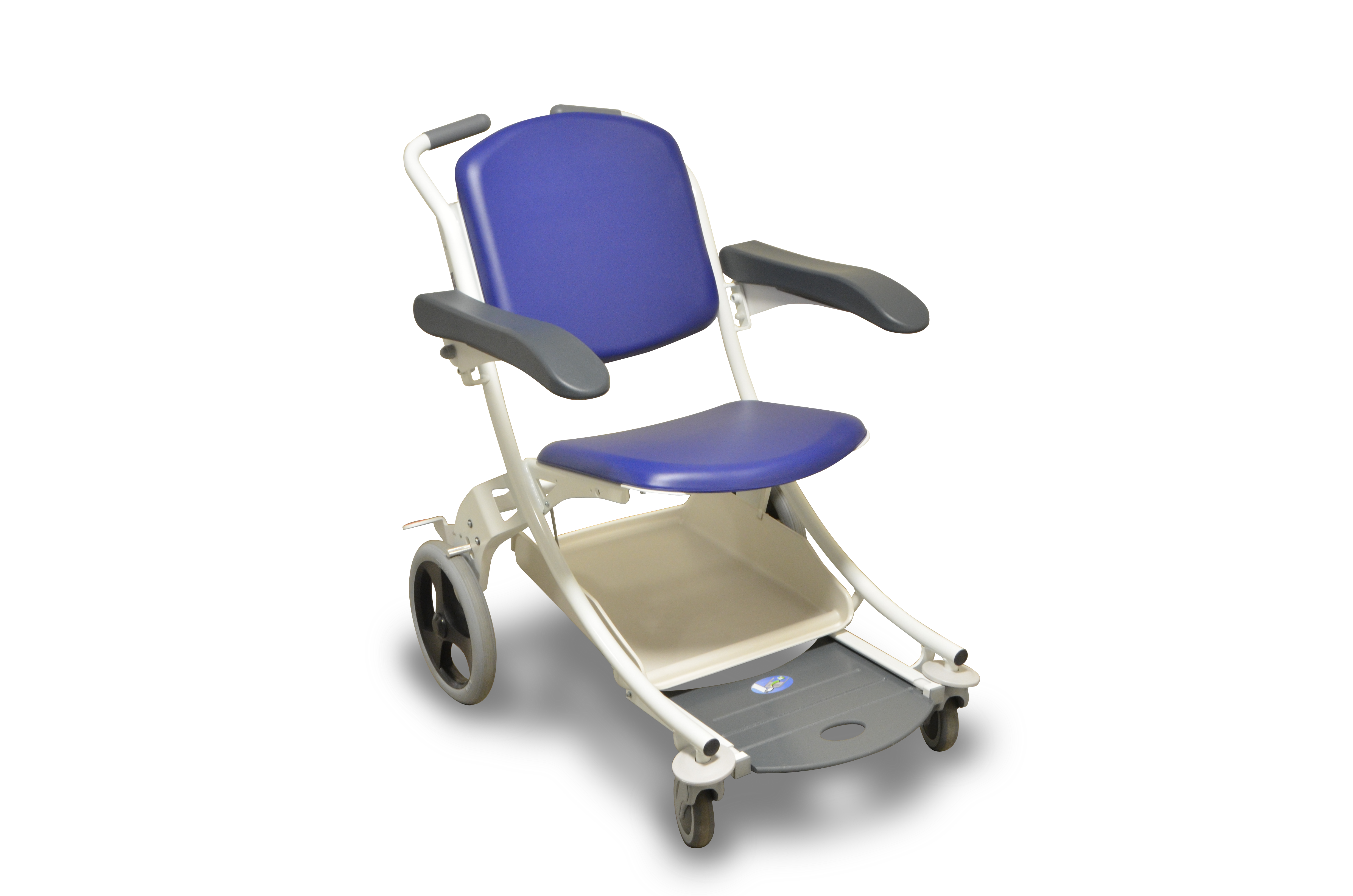
Hospital teams have told us about the many frustrations they experience when it comes to managing wheelchairs for patient transfers. We've listened to your need and are excited to bring the I-MOVE to New Zealand.
A popular solution in European hospitals, the I-MOVE is a quality transfer chair that will make life easier and provide a better experience for patients of all shapes and sizes.
Manufactured in Europe by BMB Medical, -I-Move chairs are made to last. Plus, there are security/locking options that help ensure they stop vanishing from your department.
Key features
-
User-friendly features - sliding footrest and cushioning foam seat make it comfortable for patients;
-
Fold-up armrests to support safe transfers;
-
For patient safety - central braking system easily operated from a standing position by the attendant;
-
Front swivel castors ensure the I-MOVE moves seamlessly;
-
Space-saving solution - chairs stack neatly together when not in use;
-
Can be locked for additional security if required - no more missing chairs!
-
Easy to clean with superior infection control properties.
For more information, contact Cubro: Email: hello@cubro.co.nz or telephone 0800 656 527. Alternatively go to: https://cubro.co.nz/products/mobility-equipment/i-move-transfer-chair-3186
Biodegradable holloware from Bamford

WM Bamford & Co Ltd has introduced a range of plant-based biodegradable medical hollowware to the New Zealand Market.
Items currently include a kidney dish, gallipot, injection tray and anaesthetic tray plus also a pleated paper pill cup, a medication cup, denture cup with lid and individually wrapped bendy white drinking straws.
Available under the EcoAid® brand name, these eco-friendly, sustainable products are made from sugarcane fibre, a readilyrenewable resource which is disposable, compostable and biodegradable. Sugarcane is a readily renewable resource and can beturned into products normally made from plastic and paper. EcoAid is dedicated to reducing the plastic footprint and negative impacts on our environment and hopes that one day all single-useand disposable medical plastic ware will be replaced with biodegradable and environmentally friendly material.
The EcoAid® range is puncture-resistant, waterproof with no plastic or wax lining and can be used for hot or cold items.
These products comply with ASTM D6868 and EN13432 for compostability. For more information about the EcoAid® range,contact Bamfords: 0800 226 3673 or email: enquiries@bamford.co.nz
Water-Jel Radiotherapy treatment gels
Water-Jel Technologies has been offering tried and trusted emergency burn care products for the pre-hospital treatment of burn injuries for 25 years. As the world market leader, it was the first to introduce the water-based gel technology that revolutionized emergency first aid for burns. Water-Jel's R1 Cooling Jel is the first step of the full R1 & R2 treatment. It cools, relieves pain and hydrates the skin. When R1 is applied to the skin it provides immediate hydration. R1 includes Lactokine in its formulation, which contains milk-derived protein and factors that assist skin to maintain a smooth and consistent texture.
The second step of the full R1 & R2 treatment is the R2 Soothing Lotion. It revitalizes, strengthens and reinforces the skin's natural defences by promoting its rapid regeneration. R2 hydrates and soothes the skin damaged by radiation.
R1 and R2 are sold as a combination pack and as separate products through Jackson Allison - tel: 0800 333 103 or 09 622 2277; E-mail: enquiries@jackson-allison.co.nz
O&M Halyard now with EBOS
The Halyard product range is now distributed in New Zealand by EBOS Healthcare. Halyard, part of the Owens & Minor family since 2018, is focused on advancing health and healthcare by delivering clinically-superior infection prevention and surgical solutions. Halyard sells its recognized sterilisation wrap, facial protection gloves, protective apparel, surgical drapes and gowns in more than 90 countries, and holds leading market positions across the portfolio.
Formerly part of Kimberly-Clark, Halyard Health became an independent company headquartered in Alpharetta, Georgia in 2014. Halyard operates 11 global manufacturing facilities with 12,700 employees worldwide – generating approximately $US1.6 billion in net revenues. In May 2018 Halyard’s Surgical and Infection Prevention business was sold to Owens & Minor of Richmond, Virginia, USA and the resultant company is now known as O&M Halyard.
Founded in 1882, Owens & Minor has annual sales in excess of $US10 billion from its global healthcare solutions, integrated technologies, products, and services provided in 90 countries. In New Zealand, the Halyard range is distributed by EBOS Healthcare.
EBOS has its roots in Christchurch where it started business under the name Early Brothers Trading Co. Ltd, selling lamps for horse drawn carriages.
In 1954 the company's name is changed to Early Bros Dental & Surgical Supplies Ltd. and in 1960 the company listed on the New Zealand stock exchange. In 1986 the company name changed to EBOS Group Ltd. As a single source supply business with distribution coverage across Australia, New Zealand and the Pacific Islands, EBOS Healthcare is well positioned to service its customers for all their health and medical product requirements throughout Australasia.
Enquiries on the Halyard product range should be addressed to: Michelle Humphries; Email: mhumphries@ebos.co.nz; Tel: 0800 18 17 16 or mobile: 021 394 767.
Vapor-Clean Filters for MH-Susceptible patients
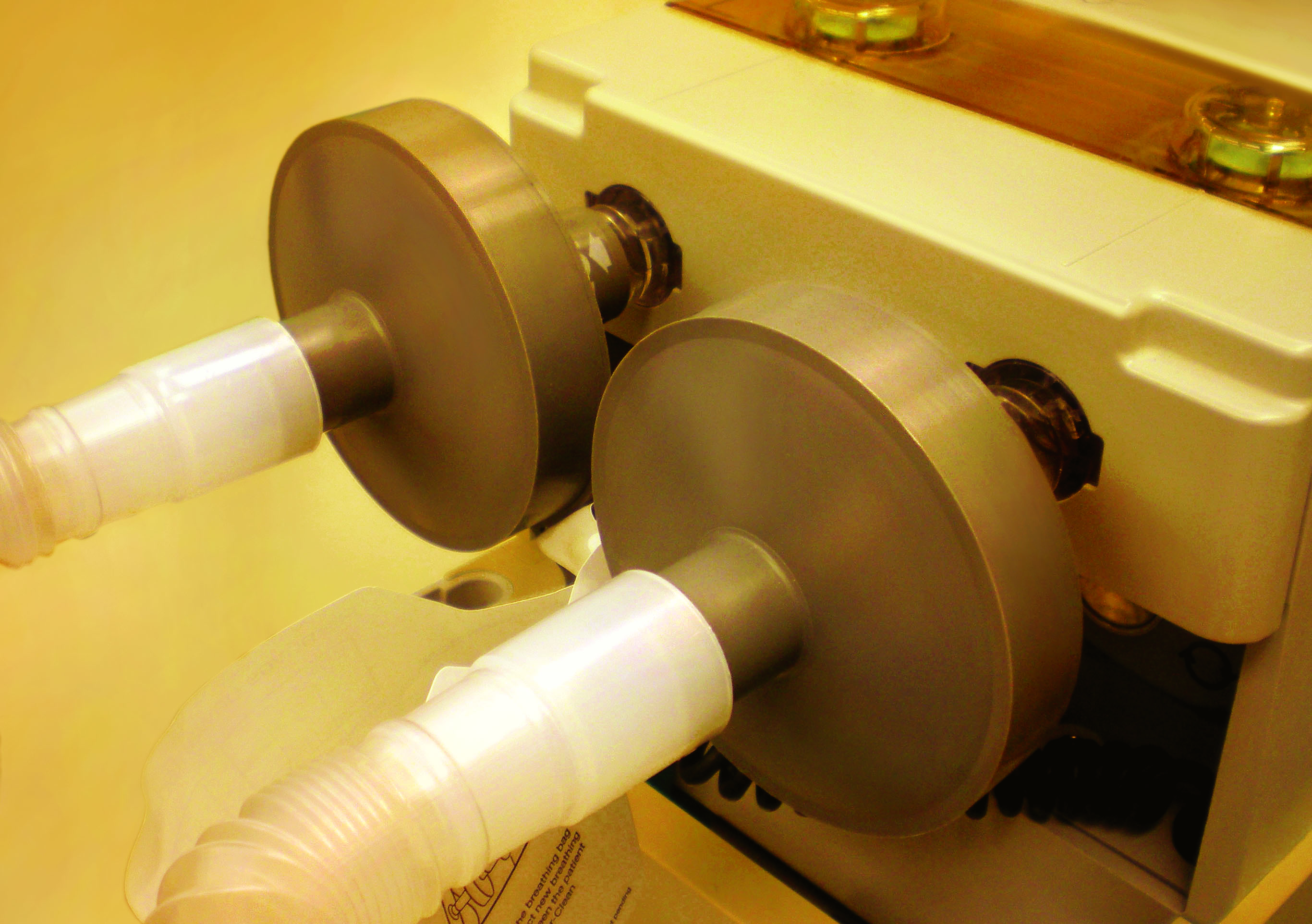
Newer anesthesia gas machines contain plastic and elastomeric components that absorb volatile anesthetics, then release residual vapor during subsequent anesthetic procedures. They require high O2 flows and a lengthy time period to remove most of the vapour before the machine can be used for a patient who cannot tolerate breathing even trace amounts of volatile anesthetic vapour.
Dynasthetics Vapor-Clean filters are for use with patients susceptible to malignant hyperthermia (MH) and patients, undiagnosed, who present with MH symptoms during anaesthesia.
Current guidelines are for a 60 minute O2 flush at 10 litres/minute due to the multiple plastic components that absorb volatile anaesthetic vapours.
The Vapor-Clean filters absorb the trace amounts of anesthetic vapor (isoflurane, sevoflurane and desflurane) so that anesthetic vapors do not reach the patient. Placement of the Vapor-Clean filter canisters on the anesthesia machine allows the machine to be vapour-free (less than five parts per million of vapour) in 90 seconds.
After one hour, replace the Vapor-Clean filters with a new pair.
For more information, contactJackson Allison Medical & Surgical Ltd (JAMS) customer service desk– Freephone: 0800 333 103;Email: enquiries@jackson-allison.co.nz; Website: https://jacksonallison.nz/assets/uploads/2016/07/Vapour-Clean-brochure.pdf
Jackson Allison's Vermed range

Jackson Allison is also the New Zealand distributor of the Nisha Medical International Inc. range of Vermed products: electrode sensors, ECG electrodes, ultrasound films and gels, defib pads, grounding pads and medical chart paper. The powerful combination of US-based manufacturer Vermed’s rich history (dating back to 1909 as the original chart paper manufacturer) and Nissha’s diverse manufacturing capabilities continue to foster tremendous growth have made Vermed an industry leader.
Contact Jackson Allison, tel: 0800 333 103 or 09 622 2277; E-mail: enquiries@jackson-allison.co.nz
Ostomy care range from Trio Healthcare
Trio Healthcare has a range of ostomy care products, distributed in New Zealand by Jackson Allison. Trio’s advanced silicone technology medical adhesive offers many benefits over traditional and rather outdated hydrocolloid medical adhesive and ostomy devices. Ostomy care applications require robust medical adhesives that maintain adhesion in a challenging environment and in a location on the body that is seldom flat. Conventional silicone gels (as seen in wound care) do not allow the skin to breathe. However, Trio’s custom formulation of silicone gel medical adhesives overcome the limitations of conventional silicone gels.
Contact Jackson Allison, tel: 0800 333 103 or 09 622 2277; E-mail: enquiries@jackson-allison.co.nz